PTH, Intact and Ionized Calcium
Main Content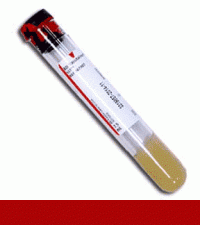
Test Code: 36736
CPT Code(s): 82310, 82330, 83970
Includes: PTH Intact, Calcium, Ionized Calcium
Methodology: PTH Immunoassay (IA) • Calcium: Spectrophotometry (SP) • Ionized Calcium: Ion Specific Electrode (ISE)
Clinical Significance: The hormone Parathyroid Hormone (PTH) acts to increase serum calcium and 1-, 25- dihydroxyvitamin D concentrations, while decreasing phosphorus. Cross-reactivity with fragment 7-84 may occur in patients with renal insufficiency. The BioIntact PTH assay is considered the most reliable. Parathyroid function is related to the calcium concentration so both results should be interpreted together.
The assay is useful in making the diagnosis of primary hyperparathyroidism, secondary hyperparathyroidism, and a differential diagnosis of hypercalcemia. The assay helps in distinguishing hypercalcemia caused by either primary hyperparathyroidism or malignant disease.
Additional names: 3rd Generation PTH, Immunoreactive PTH, Intact Hormone, Parathyroid Hormone, PTH Whole Molecule, Sandwich PTH Assay, Two Site Antibody Assay, Intact PTH & CA, Monogram, Parathyroid, PTH, PTH Assay, PTH, Intact, PTH, Intact(Irma) & Calcium
Calcuim, Ionized
The Ionized Calcium is determined by an ion selective electrode methodology. The result that is generated is Ph adjusted. The result is empirically based on a measured Ph and Ionized Calcium concentration normalized to a Ph of 7.40. This calculation compensates for in-vitro changes in Ph due to loss of co2 through specimen handling. Ionized calcium represents the true "bioavailable" calcium in the circulation. In situations where the total calcium is normal but does not fit the clinical picture, e.g., Hyperpara-Thyroidism, a determination of the ionized calcium will, many times, show an elevation in the "bioavailable" calcium component. This may be due to alterations in protein concentrations, especially albumin, which binds most of the calcium in the circulation.
Two seperate specimens vials needed.
2ml frozen serum tube and one unopened spun SST tube
Supply: T01 - Red/Gray SST 8.5mL
Test Code: 36736
CPT Code(s): 82310, 82330, 83970
PTH Intact
Preferred Specimen Requirements: 2 mL Frozen Serum
Instructions: Spin to separate serum and transfer to plastic screw-cap vial. Freeze immediately and submit to laboratory frozen. Do not submit glass tubes.
Note: Sodium or lithium heparin are no longer acceptable specimen types.
Container Type: Plastic Screw-Cap Vial
Special Instructions for Label: 1 mL *FRZ ONLY*
Transport Temperature: Frozen
- Frozen: 6 Months
- Due to Hemolysis
- Due to Lipemia
- Thawing or Any Other Rejections
- Plasma
- Received Room Temperature
- Received Refrigerated
Calcium, Ionized
Preferred Specimen Requirements: 2 mL serum collected in a gel barrier tube.
Instructions: Let clot and spin immediately with the cap on. Do not open tube. Ship the unopened gel barrier tube at room temperature. If submitting with any other assay, please submit a separate tube for this test.
Container type: Unopened gel barrier tube other acceptable specimen requirements SST (red/gray-top) tube
Special instructions for label: *******Do Not Open*********
Transport temperature: Room temperature
- Room temperature: 1 week
- Refrigerated: 1 week
- Hemolysis
- Lipemia
- Received frozen
For additional supply or collection device information, please contact DLO's Customer Service at (800) 891-2917, option 2.
The CPT codes provided are based on AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the payor being billed.


CLIA