D-Dimer
Main Content
Test code: 8659
CPT code(s): 85379
Methodology: Photo-optical
Clinical Significance: Venous thromboembolism (VTE) is associated with significant morbidity and mortality, and has two patterns of clinical presentation, deep venous thrombosis (DVT) and pulmonary embolism (PE). It is an important medical problem with an estimated incidence in the United States of 100,000 to 300,000 cases per year. Diagnosis relies on objective testing, as the clinical signs and symptoms of the disease are nonspecific. Various invasive and noninvasisve diagnostic procedures have been developed, but none are ideal.
D-Dimer is generated fromt he plasmin mediated lysis of cross-linked fibrin. Increased levels of D-Dimer indicate that both the procoagulant (thrombin generating pathways) and fibrinolytic systems (plasmin generating pathways) have been activated. Venous thrombosis activates both the coagulation and fibrinolytic pathways and results in elevated levels of fibrin degradation products, such as D-Dimer.
Unfortunately, an elevated D-Dimer level is not specific for VTE. There are many other situations that will yield increased levels, such as cancer, trauma, prganancy, inflammation, surgery, advancing age, and extended bed-rest. However, it is extremely unlikely that a paitent with an acute VTE will have D-Dimer levels in the normal range. Therefore, the clinical utility of the D-Dimer assay is its ability to assist in "ruling out" a diagnosis of VTE.
Includes: D-Dimer
Alternative Name(s): N/A
Supply: T04 - Light Blue 2.7mL
Preferred Specimen: One Full Unopened 3.2% Sodium Citrate (Light Blue-Top) Tube
Preferred Volume: 2.7mL
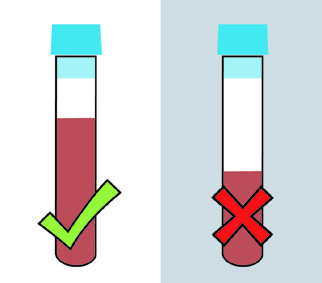
A completely filled tube is necessary because the correct ratio of blood to citrate is critical (9:1). Mix by gentle inversion 3-4 times. Do not uncap.
Instructions: a completely filled tube is necessary because the correct ratio of blood to citrate is critical (9:1). Mix by gentle inversion 3-4 times. Do not uncap. Minimum Volume Ratio of blood to citrate is critical.
Coagulation specimen collection requirements:
- A light-blue top tube (containing 3.2% sodium citrate) that will be used for coagulation testing must be filled to completion. Please see the attached BD guides for instructions. Under-filling the tube changes the ratio of blood to anticoagulant additive. The under-filled tube will be considered an improper specimen.
- Specimen must reach the band etched into the tube – it is critical that that band is reached by the specimen. The position of the label and any markings on it are immaterial – that etched band is the goal.
- If a winged blood collection device (butterfly) is used to collect a light-blue top tube for coagulation studies, a waste tube must be drawn first. The waste tube must also be a light-blue top tube or a tube that contains no additives. This waste tube is drawn first to remove the air in the tubing of the winged collection device. Once blood flows through the tubing, the waste tube can be removed and discarded. The waste tube does not need to be completely filled. If the air is not displaced from the tubing into a waste tube, it will be drawn into the specimen tube and cause a short-fill of the tube. Less volume of blood in the tube alters the required blood-to-anticoagulant ratio needed for coagulation studies. The under-filled tube will be considered an improper specimen.
- Expired tubes lose their vacuum and will not pull enough blood into the tube for the correct blood-to-anticoagulant ration. The under-filled tube will be considered an improper specimen.
Transport Container: 3.2% Sodium Citrate (Light Blue-Top) Tube
Transport Temperature: Room Temperature
Specimen Stability: Room Temperature: 72 Hours
Reject Criteria:
- Improper Blood To Citrate Ratio
- Clotted
- Grossly hemolyzed specimens
- Improper Blood Collection
For additional supply or collection device information, please contact DLO's Customer Service at (800) 891-2917, option 2.
The CPT codes provided are based on AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the payor being billed.


CLIA