COVID, Flu A/B Panel
Main Content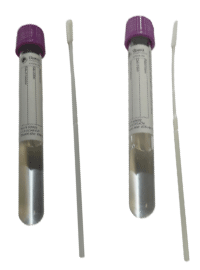
Test code: 97637
CPT code(s): 87635 (HCPCS: U0003. U0005 will be added at an additional charge consistent with applicable payer requirements), 87804 (x2)
Methodology: Nucleic Acid Amplification Test (NAAT) include PCR or TMA, Optical Immunoassay
Includes: SARS-CoV-2 (COVID-19) and Influenza A & B
Clinical significance:
SARS-CoV-2 RNA (COVID-19), Qualitative NAAT - The SARS-CoV-2 RNA (COVID-19), Nucleic-acid Amplification Test (NAAT) is a qualitative multi-target molecular diagnostics test that aids in the detection of COVID-19. This test is intended to be performed on respiratory specimens collected from individuals who meet the Centers for Diseases Control and Prevention (CDC) clinical and/or Epidemiological criteria for COVID-19 testing. For details visit: https://www.cdc.gov/coronavirus/2019-ncov/hcp/index.html.
Influenza is an acute viral disease that is seasonal in incidence, occurring in the colder months. The illness classically presents with sudden onset of fever, chills, headache, myalgia and a non-productive cough. Influenza A or B virus cause the majority of clinically significant disease, with influenza C virus being responsible only for mild, predominately upper respiratory tract illness. Patients who present with suspected influenza may benefit from treatment with antiviral agents. Since the therapeutic options have expanded to include options for the treatment of influenza B disease, it is important to rapidly distinguish influenza A from influenza B in order to allow physicians a choice in selective antiviral intervention. This will also allow for the appropriate preventative intervention to be taken in institutions where measures can be taken for susceptible individuals. It is therefore important to not only rapidly determine whether influenza is present, but also which type of influenza virus is present.
Supply: NP: K162; AN: K165 KIT (tube top colors may vary)
Preferred specimen(s): Nasopharyngeal (NP) swab OR Anterior Nasal swab
Preferred volume:1 swab
Transport container:
Transport temperature: Refrigerated (cold packs)
Specimen stability: Refrigerated: 72 hours
Rejection Criteria: Amies Gel Bacterial Transport Swab; Specimens received on calcium alginate swabs and swabs other than dacron, rayon or flocked nylon; Swabs with shafts other than plastic or aluminum; Specimens received at room temperature (unless the testing is done at point of care)
For additional supply or collection device information, please contact DLO's Customer Service at (800) 891-2917, option 2.
The CPT codes provided are based on AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the payor being billed.


CLIA