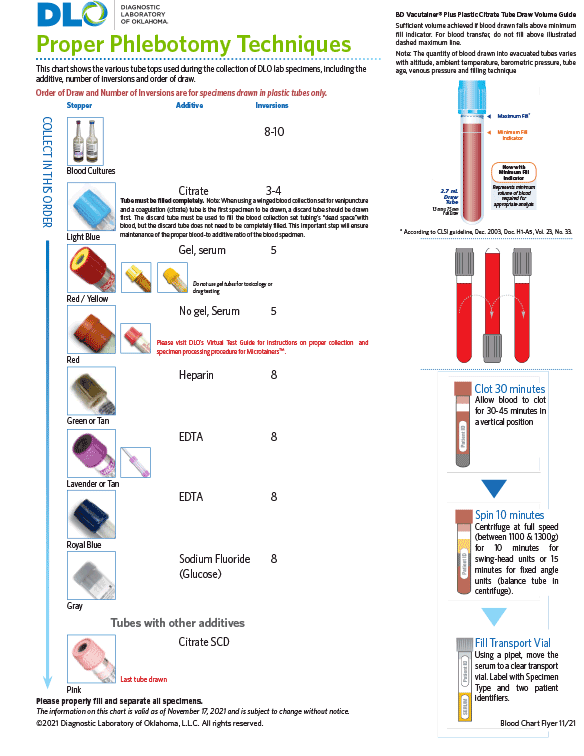
Blood Specimen Collection
Download DLO's Blood Specimen Collection Chart
For additional supply or collection device information, please contact DLO's Customer Services at (800) 891-2917, option 2.
For additional supply or collection device information, please contact DLO's Customer Services at (800) 891-2917, option 2.
Test Code: 97714
CPT Code: 87497
Alternative Name(s): CMV DNA QN PCR
Methodology: Polymerase Chain Reaction
Test Code: 97642
CPT Codes: 87481 (x5)
Pathogens detected: Candida albicans, Candida glabrata, Candida krusei, Candida parapsilosis, Candida tropicalis
Disclaimer: The Sexually Transmitted Disease Panels are not recommended for evaluation of suspected sexual abuse or for other medico-legal indications.
Test code: 15059
Methodology: Centrifugation enhanced culture using R-mix cell line
Includes: Respiratory syncytial virus (RSV)
Alternative name(s): RSV Rapid culture, Respiratory Syncytial Virus Rapid Culture
Test code: 16796
Methodology: Chemiluminescence (CL)
Clinical Significance: Calprotectin is a non-specific marker of bowel inflammation. Subsequent to white blood cell migration into the intestine, this neutrophil protein may be detected in the stool. Thus, fecal calprotectin levels may assist in diagnosis of inflammatory bowel disease; Crohn’s disease and ulcerative colitis and other disorders characterized by bowel inflammation. It can also be used as an aid in the differentiation of IBD from irritable bowel syndrome.
Test Code: 5509
CPT Code: 82140
Alternative Name(s): Not applicable
Methodology: Enzymatic
Test Code: 443
CPT Code(s): 80320
Includes: Alcohol, Ethyl
Methodology: Alcohol dehydrogenase
Alternative Name(s): Alcohol, Ethyl
Test Code: 91734
CPT Code: 82542
Methodology: Chromatography/Mass Spectrometry
This test was developed and its analytical performance characteristics have been determined by Quest Diagnostics Nichols Institute, San Juan Capistrano, CA. It has not been cleared or approved by the FDA. This assay has been validated pursuant to the CLIA regulations and is used for clinical purposes.
Includes: Omega-3 (EPA+DHA) Index, Omega-6/Omega-3 Ratio, EPA/Arachidonic Acid Ratio, Arachidonic Acid, EPA, DHA
Test Code: 4212
CPT Code(s): 82533
Methodology: Immunoassay (IA)
Clinical Significance: Cortisol is increased in Cushing's Disease and decreased in Addison's Disease (adrenal insufficiency).
Alternative Name(s): N/A
Test Code: 10458
CPT Code: 81220
Alternative Name(s): CF Mutation Screen,Cystic Fibrosis Mutation Screen,CFTR Screen,CF Carrier Screen,CF Screen,Cystic Fibrosis Carrier Screen
Methodology: Multiplex Polymerase Chain Reaction • Massively Parallel Sequencing