Blood Specimen Collection
Download DLO's Blood Specimen Collection Chart
For additional supply or collection device information, please contact DLO's Customer Services at (800) 891-2917, option 2.
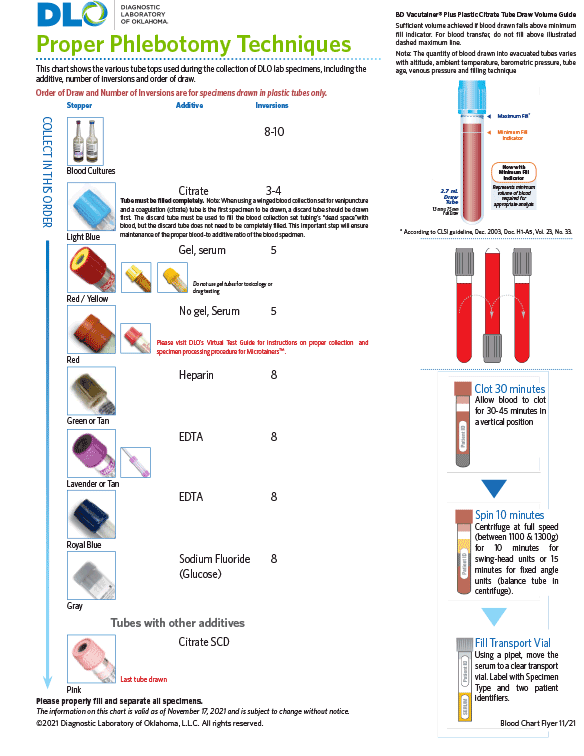
For additional supply or collection device information, please contact DLO's Customer Services at (800) 891-2917, option 2.
Test Code: 97758
CPT Code: 87536
Alternative Name(s): HIV-1 RNA QN RT-PCR
Methodology: Reverse Transcription Polymerase Chain Reaction
Test Code: 97756
CPT Code: 87522
Alternative Name(s): HCV RNA QN RT-PCR
Methodology: Reverse Transcription Polymerase Chain Reaction
Test Code: 97757
CPT Code: 87517
Alternative Name(s): HBV DNA QN PCR
Methodology: Real-time Polymerase Chain Reaction
Test Code: 97713
CPT Code: 87799
Alternative Name(s): EBV DNA QN PCR
Methodology: Polymerase Chain Reaction
Test Code: 97714
CPT Code: 87497
Alternative Name(s): CMV DNA QN PCR
Methodology: Polymerase Chain Reaction
Test Code: 97711
CPT Codes: 87481 (x5), 87491, 87529 (x2), 87563, 87591, 87661, 87798 (x5), 87511
Test Code: 97642
CPT Codes: 87481 (x5)
Pathogens detected: Candida albicans, Candida glabrata, Candida krusei, Candida parapsilosis, Candida tropicalis
Disclaimer: The Sexually Transmitted Disease Panels are not recommended for evaluation of suspected sexual abuse or for other medico-legal indications.
Test Code: 97641
CPT Codes: 87491, 87563, 87591, 87661, 87529 (x2)
Pathogens detected: Chlamydia trachomatis, Mycoplasma genitalium, Neisseria gonorrhoeae, Trichomonas vaginals, Herpes simplex virus 1 & 2
Disclaimer: The Sexually Transmitted Disease Panels are not recommended for evaluation of suspected sexual abuse or for other medico-legal indications.
Test Code: 97712
CPT Codes: 87798 (x3), 87511, 87798 (x2)
Pathogens detected: Atopbium vaginae, Enterococcus faecalis, Esherichia coli, Gardnerella vaginalis, Mycoplasma hominis, Ureaplasma urealyticum
Disclaimer: The Sexually Transmitted Disease Panels are not recommended for evaluation of suspected sexual abuse or for other medico-legal indications.