Blood Specimen Collection
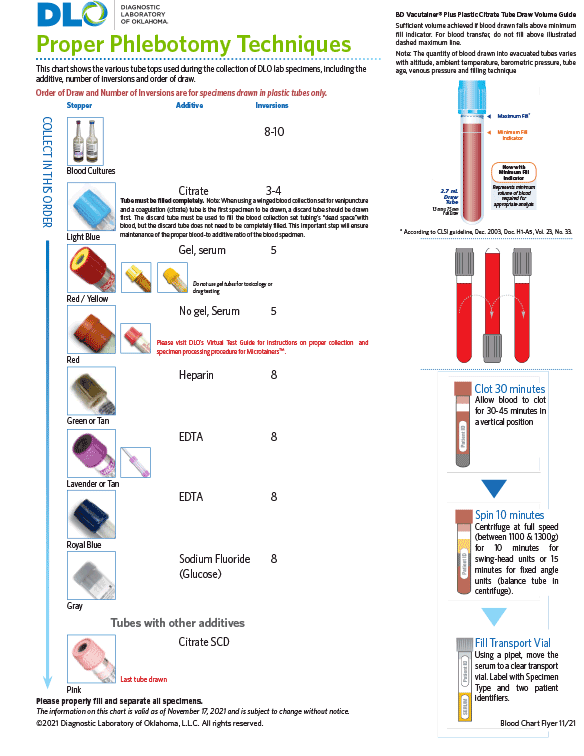
Download DLO's Blood Specimen Collection Chart
For additional supply or collection device information, please contact DLO's Customer Services at (800) 891-2917, option 2.
For additional supply or collection device information, please contact DLO's Customer Services at (800) 891-2917, option 2.
Test Code: 90569
CPT Codes: 87529 (x2)
In instances where computer-assisted Pap testing is unable to make an evaluation, manual screening will be performed. All SurePath™ Imaging Pap tests and all ThinPrep® Imaging System tests will be CPT coded as 88175.
Pap results requiring physician interpretation will be performed at an additional charge (CPT Code(s): 88141; HCPCS: G0124).
Methodology: Liquid Based-Density Gradient Sedimentation
Test Code: 90521
CPT Codes: 87661
In instances where computer-assisted Pap testing is unable to make an evaluation, manual screening will be performed. All SurePath™ Imaging Pap tests and all ThinPrep® Imaging System tests will be CPT coded as 88175.
Pap results requiring physician interpretation will be performed at an additional charge (CPT Code(s): 88141; HCPCS: G0124).
Methodology: Liquid Based-Density Gradient Sedimentation
Test Code: 11363
CPT Codes: 87491, 87591
In instances where computer-assisted Pap testing is unable to make an evaluation, manual screening will be performed. All SurePath™ Imaging Pap tests and all ThinPrep® Imaging System tests will be CPT coded as 88175.
Pap results requiring physician interpretation will be performed at an additional charge (CPT Code(s): 88141; HCPCS: G0124).
Methodology: Liquid Based-Density Gradient Sedimentation
Test Code: 11362
CPT Code: 87591
In instances where computer-assisted Pap testing is unable to make an evaluation, manual screening will be performed. All SurePath™ Imaging Pap tests and all ThinPrep® Imaging System tests will be CPT coded as 88175.
Pap results requiring physician interpretation will be performed at an additional charge (CPT Code(s): 88141; HCPCS: G0124).
Methodology: Liquid Based-Density Gradient Sedimentation
Test Code: 11361
CPT Codes: 87491
In instances where computer-assisted Pap testing is unable to make an evaluation, manual screening will be performed. All SurePath™ Imaging Pap tests and all ThinPrep® Imaging System tests will be CPT coded as 88175.
Pap results requiring physician interpretation will be performed at an additional charge (CPT Code(s): 88141; HCPCS: G0124).
Methodology: Liquid Based-Density Gradient Sedimentation
Test Code: 18829
CPT Codes: 88175 (HCPCS: G0145), 87624
In instances where computer-assisted Pap testing is unable to make an evaluation, manual screening will be performed. All SurePath™ Imaging Pap tests and all ThinPrep® Imaging System tests will be CPT coded as 88175.
Pap results requiring physician interpretation will be performed at an additional charge (CPT Code(s): 88141; HCPCS: G0124).
Methodology: Liquid Based-Density Gradient Sedimentation
Test Code: 18828
CPT Codes: 88175 (HCPCS: G0145), 87624, 87491, 87591
In instances where computer-assisted Pap testing is unable to make an evaluation, manual screening will be performed. All SurePath™ Imaging Pap tests and all ThinPrep® Imaging System tests will be CPT coded as 88175.
Pap results requiring physician interpretation will be performed at an additional charge (CPT Code(s): 88141; HCPCS: G0124).
Methodology: Liquid Based-Density Gradient Sedimentation
Test Code: 18813
CPT Code: 88175 (HCPCS: G0145), 87624
In instances where computer-assisted Pap testing is unable to make an evaluation, manual screening will be performed. All SurePath™ Imaging Pap tests and all ThinPrep® Imaging System tests will be CPT coded as 88175.
Pap results requiring physician interpretation will be performed at an additional charge (CPT Code(s): 88141; HCPCS: G0124).
Methodology: Liquid Based-Density Gradient Sedimentation
Test Code: 18811
CPT Code: 88175 (HCPCS: G0145)
In instances where computer-assisted Pap testing is unable to make an evaluation, manual screening will be performed. All SurePath™ Imaging Pap tests and all ThinPrep® Imaging System tests will be CPT coded as 88175.
Pap results requiring physician interpretation will be performed at an additional charge (CPT Code(s): 88141; HCPCS: G0124).
Methodology: Liquid Based-Density Gradient Sedimentation