Test Code: 17656
CPT Code(s): 87641
Mehodolgy: Polymerase Chain Reaction (PCR)
Reference Range(s): Not detected
Clinical Significance: This assay is used to detect the presence of Methicillin-Resistant Staphylococcus aureus (MRSA) DNA in a patient's nasal swab specimen. The use of real-time PCR to detect the presence of MRSA DNA in clinical specimens allows for rapid patient testing.
Alternative Name(s): MRSA PCR, Staphylococcus aureus, Methicillin-resistant
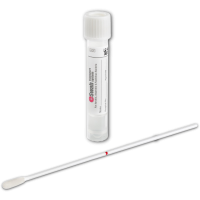
Supply: #S12 ESwab™ Specimen Collection and Transport System - White Cap
Collect nasal specimens according to the following procedure using the recommended swab.
1. Moisten the swab with two drops (about 50 uL) of sterile physiological serum (saline) or use it dry.
2. Carefully insert the swab into the patient’s nostril. The swab tip must be inserted up to 2.5cm (1 inch) from the edge of the nares. Roll the swab along the mucosa inside the nostril 5 times.
3. Insert the same swab into the second nostril and repeat sampling as in step 2.
4. Replace the swab in its transport tube. Label the transport tube with two patient identifiers.
Minimum volume: One swab
Transport container: Nasal Swab
Transport temperature: Refrigerated (cold packs)
Specimen Stability: Refrigerated: 5 days
Reject criteria:
- Frozen specimens
- Specimens exceeding stability
- Specimens in leaking or broken containers
- Non-validated specimen types
- Wire swabs
For additional supply or collection device information, please contact DLO's Customer Service at (800) 891-2917, option 2.
The CPT codes provided are based on AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the Payor being billed.
