Test code: 90801
CPT code(s): 87661
Preferred specimen(s):
- Aptima urine collection kit (yellow label)
- Aptima unisex swab specimen collection kit (white label)
Reference Range(s):
T. vaginalis RNA, QL, TMA, Males - Not detected
This test was performed using the APTIMA® Trichomonas vaginalis assay (GEN-PROBE®).
Clinical Significance: This test is used to detect Trichomonas vaginalis in clinical specimens. The test has greater analytical sensitivity than culture methods.
Test FAQ: SureSwab® Trichomonas vaginalis RNA, Qualitative TMA
Alternative Name(s): T. vaginalis, Urine Trichomonas, Trichomonas Urine
Methodology: Transcription Mediated amplification (TMA)
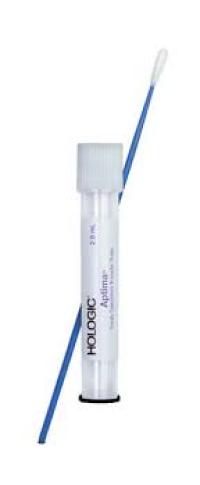
Supply: #A02 APTIMA® Unisex Collection
Additional Tests with APTIMA Unisex as Preferred Specimen Colleciton Device:
11363, CT/NG
10121, SureSwab® Advanced Candida Vaginitis (CV), TMA
Preferred Specimen: Male Urtheral Swab Specimen
Patient should not have urinated within an hour prior to specimen collection.
Discard cleaning swab (white shaft with red print on label). The cleaning swab if NOT needed for male specimen collection. Insert specimen collection swab (blue shaft swab in package with green printing) 2 cm to 4 cm into urethra. Gently rotate swab clockwise for 2 to 3 seconds in urethra to ensure adequate sampling. Withdraw swab carefully.
While holding swab in hand, unscrew the tube cap. Do not spill tube contents. If the tube contents are spilled, discard and get a new APTIMA Unisex swab transport tube. Immediately place swab into transport tube so that the tip of the swab is visible below tube label. Carefully break swabs shaft at the score line against the side of the tube. Discard top portion of swab shaft.
Re-cap swab specimen transport tube tightly.
Transport Container: Aptima® transport tube
Transport Temperature: Room temperature
- Room temperature: 60 days
- Refrigerated: 60 days
- Frozen: 6 months
For additional supply or collection device information, please contact DLO's Customer Service at (800) 891-2917, option 2.
The CPT codes provided are based on AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the payor being billed.
